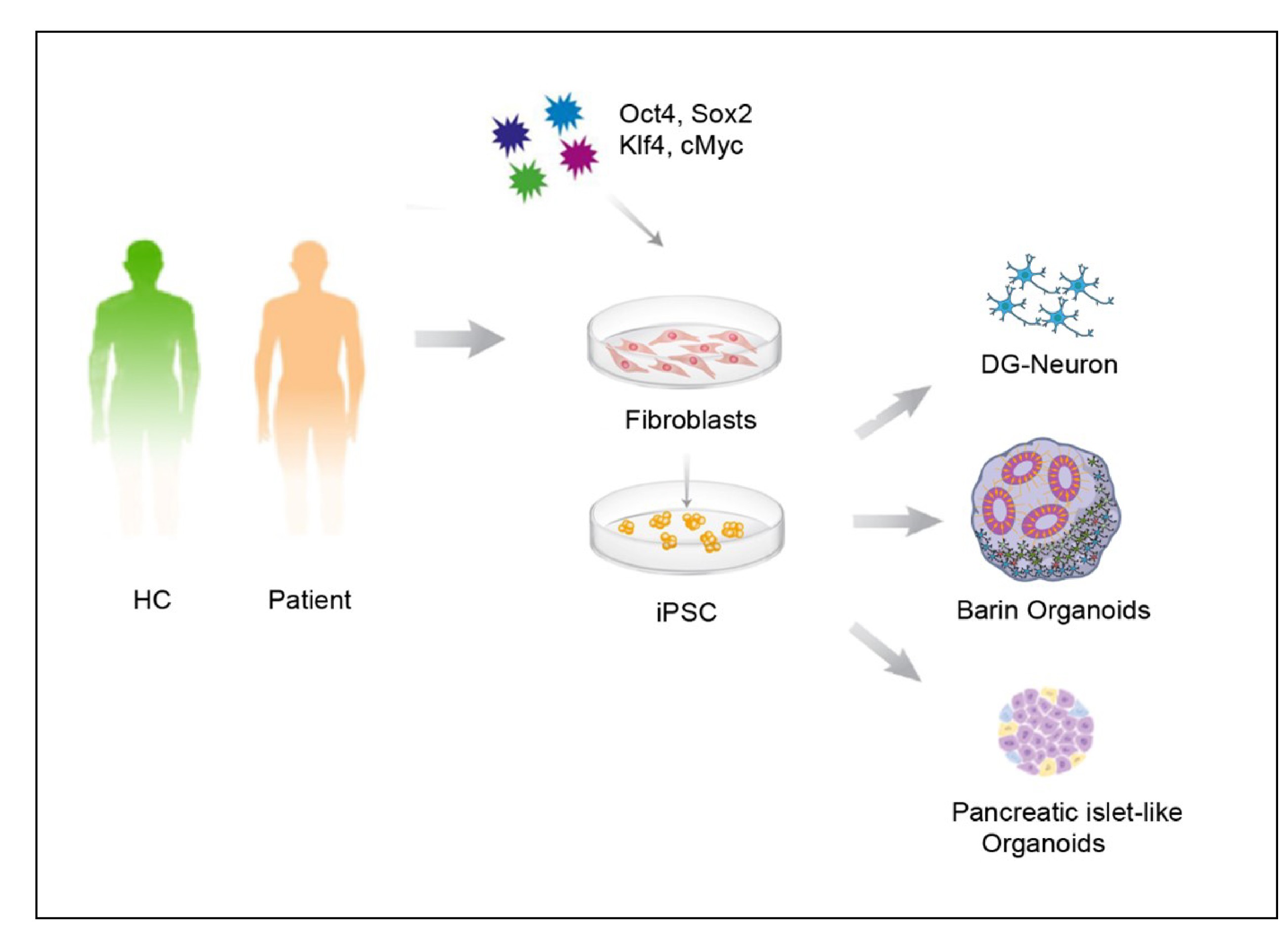
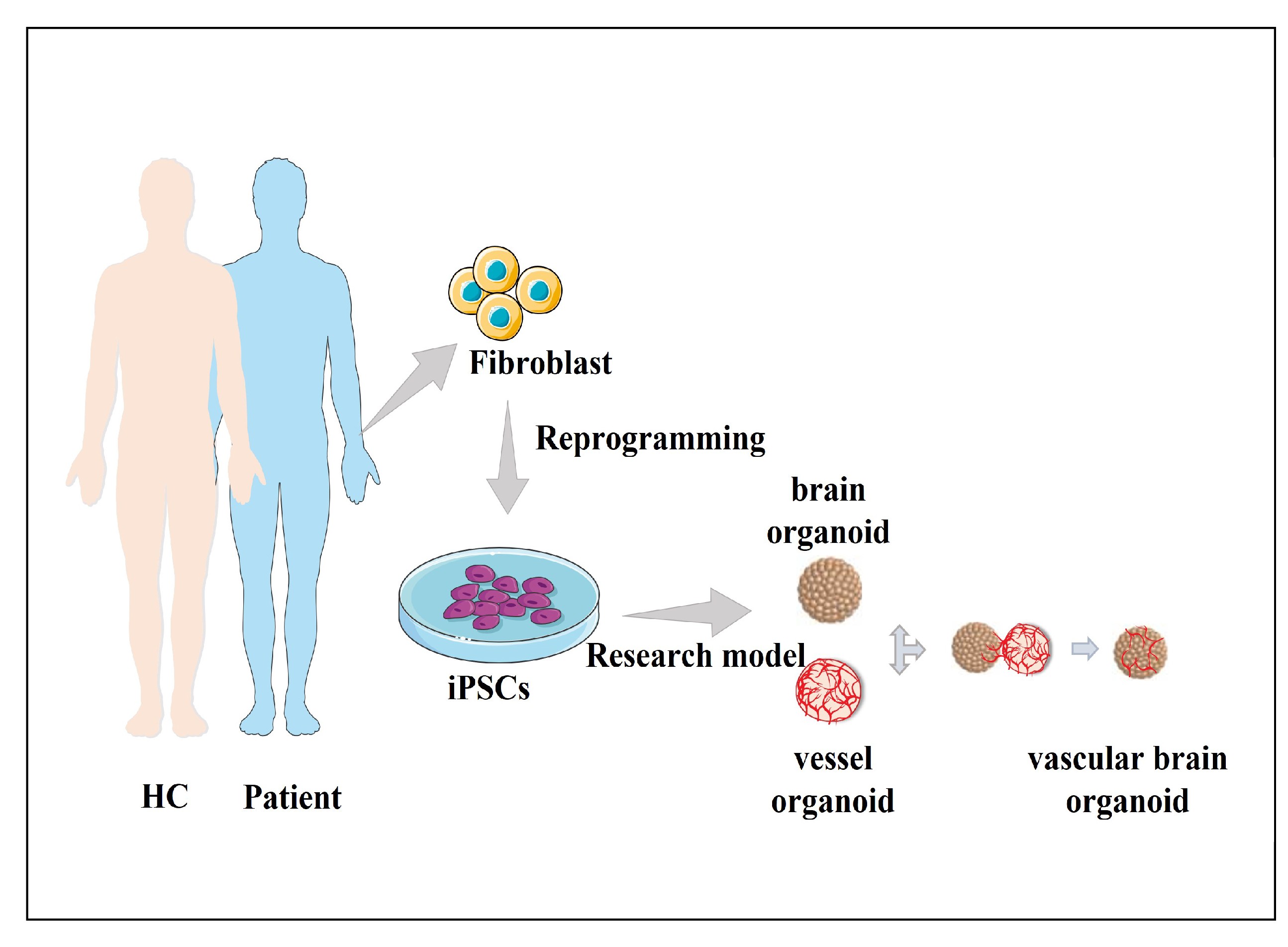
Our mechanistic research mainly focuses on bipolar disorder (BD) and depression. BD is characterized by alternating episodes of depression and mania, making it more complex than unipolar depression, autism, or schizophrenia. Consequently, the mechanistic understanding of BD remains limited.
To address this, we generate iPSC- and organoid-based models from sporadic BD patients. Through molecular genetics, electrophysiology, and fluorescence imaging, we aim to identify high-risk genetic factors across diverse patient backgrounds and to elucidate their shared molecular and cellular mechanisms.